
A Balanced Aquarium – Testing and Analysis
Introduction
The aquarium: A Natural Biological Process of Self-Regulation:
An aquarium is a stable, balanced, living environment, virtually autonomous in its biologically self-regulating processes.
With water testing technology, you can monitor and manage changes in key chemical and biological parameters in your aquarium water with peace of mind. The most common tests are: the pH and water hardness (GH/KH), and, for the biological aspects, levels of ammonia, nitrites and nitrates.
Many other more specialized tests are available on the market and are used by freshwater, saltwater and reef aquarium enthusiasts. Our goal in this brief manual is to provide the essential information you need to start and manage a traditional aquarium set-up, worry-free.
It is advisable to check, or have checked by a specialist, the parameters of your aquarium at least once every three months, if everything is going well. Several brands of tests that are practical, reliable and easy to use are available on the market.
The Biological Balance of the Aquarium: The Nitrogen Cycle
For an aquarium to operate flawlessly – without any imbalances or disease, and the ensuing maintenance headaches – it is imperative when you start a new tank to let a natural biological process called “the nitrogen cycle” establish itself.
Nitrogen is essential to all biological life, it is present in the aquarium in the form of ammonia, nitrites and nitrates (NH3/NO2/NO3).
In a balanced aquarium, waste and organic materials released by fish and plants decompose and generate ammonia as an initial by-product. Some bacteria then absorb the ammonia and transform it into nitrites and these are finally converted into nitrates by other families of bacteria. The final compound (nitrate) is not toxic at low levels and its concentration over time will be regulated mainly by regular partial water changes.
When installing a new aquarium, about five weeks are required for this biological chain to naturally get up and running and then continue in an endless loop in the aquarium. It is possible to reduce this time by adding some bacterial and enzymatic strains at the outset (see your aquarium specialist).
NH 3/NH 4+ (ammonia): The First Stage of the Nitrogen Cycle
Ammonia (NH3) is a toxic gas composed of nitrogen and hydrogen produced by the decomposition of organic matter (fish waste and plants) in the first phase of the biological nitrogen cycle. With the support of nitrifying bacteria (Nitrosomonas and Nitrospira), ammonia (toxic) turns into nitrites (toxic) and then nitrates (non-toxic at low concentrations); this is the final phase of the perpetual nitrogen cycle.
Ammonia or Ammonium?
In an aquarium, the transformation of ammonia NH3 (toxic) into ammonium NH4 (an ionic form of ammonia) is directly dependent on the acidic or basic pH ± value of the water.
- At an alkaline pH (> pH 7), a gaseous form of ammonia is present – it is highly aggressive and toxic to the plant and animal life in the aquarium.
- At a neutral or acid pH (pH <7), ammonia is chemically converted into ammonium, which is 100 times less toxic and can be assimilated by plants.
It is therefore very important to check the ammonia concentration and pH of the water, especially in the initial phase of an aquarium to confirm that the life cycle is off to a good start. The most common NH4 test is a simple colorimetric test, easy to do yourself. The level must not to exceed 0.25 mg/L, especially in freshwater that tends to be basic (high pH)
What should be done if there’s too much ammonia?
- In an aquarium that you’re just setting up, seed the filter immediately with a bacterial strain (see commercial products); feed sparingly if the tank already has fish; aerate the tank heavily and wait for the biological cycle to get on a firm footing.
- In an aquarium that’s been in operation, investigate the causes of the problem: too much food? too many fish? an unsuitable filter that’s clogged, or cleaned too often? a lack of aeration? Verify that the filter is operating properly, add a dose of zeolite (ZEOCLEAR®) ) in the filter, change one third of the volume of water, siphoning any waste on the bottom of the tank, aerate the tank and reseed the aquarium immediately with a bacterial and enzymatic strain.
Reduce the amount of food regularly provided each day by 50% and monitor the development of the cycle.
NO2 (nitrites): The Second Stage of the Nitrogen Cycle
In a well-established cycle, the nitrite ion (derived from the processing of toxic ammonia) is a compound which degrades naturally and transforms into a (nontoxic) nitrate ion through Nitrospira bacteria (final phase of the perpetual nitrogen cycle).
A significant level of nitrites in a tank is detrimental to the flora and fauna of an aquarium, and is evidence that the biological balance is either broken or has not yet gotten started. It is therefore very important to regularly check the concentration of nitrites, particularly during the first two months of the start-up phase of an aquarium.
In a balanced aquarium, even a slight increase in the rate of nitrites should be taken very seriously; it indicates that a biological imbalance is taking hold.
The NO2 test is a simple, reliable colorimetric test, very easy to do yourself. The warning threshold for NO2 is 0.15 mg/L, the toxicity threshold not to be exceeded is 0.25 mg/L, and fatal short-term threshold is 3 mg/L.
What should be done if the nitrite concentration is too high?
- In an aquarium that you’re just setting up, seed the filter immediately with a bacterial strain (see commercial products); feed sparingly if the tank already has fish; aerate the tank heavily and wait for the biological cycle to get on a firm footing before adding any fish.
- In an aquarium that’s been in operation, investigate the causes of the problem: too much food? too many fish? hidden dead plant or animal matter? a filter that’s clogged or cleaned too often? a lack of aeration? etc.). Verify that the filter is working properly, add a dose of RESIFILT Cleanwater® change one third of the volume of water, siphoning any waste on the bottom, reseed the filter with a bacterial strain and aerate the tank heavily.
Reduce the amount of food regularly provided each day by 50% and monitor the nitrite level each day.
NO3 (nitrates): The Third Stage of the Nitrogen Cycle
Produced by the breakdown of nitrites by Nitrospira bacteria, the presence of nitrates in the water analysis is the result of a proper functioning of the biological waste regeneration system in the aquarium.
Non-toxic at low doses and actually beneficial to plants as a fertilizer, the concentration of nitrates in an aquarium is constantly increased by the process of the transformation cycle and will always tend to increase. The generally accepted nitrate level for an aquarium is a maximum of 50 mg/L. Starting at a level of 100 mg/L, fish gradually weaken, plants wither and parasitic algae grow in the tank uncontrollably.
There are 3 simple rules for properly managing nitrates in the aquarium: 1) no excess food; 2) abundant vegetation; and 3) very regular partial water changes (one-quarter of the total volume monthly is a good average).
What should be done if the nitrate concentration is too high?
- Search for the causes of the problem: too much food? too many fish? absence or lack of balancing vegetation? and especially, a lack of regular partial water changes to mechanically remove the surfeit of nitrates in the aquarium.
- Verify that the filter is working properly, add a dose of RESIFILT Cleanwater® change one third of the volume of water, siphoning any waste on the bottom, reseed the filter with a bacterial strain.
Notice: if the concentration of nitrates is very high (more than 150mg/L), gradually change the water in the tank over several days (never more than 1/3 of the tank volume at a time) and continue until the nitrate level is back to reasonable values (between 50 and 100 mg/L), then go back to step 2.
If the aquarium is very old or poorly maintained, the sand is often over-saturated with silt and nitrates and it is often recommended to take the opportunity to do a complete renovation of the tank.
The Chemical Balance of the Aquarium
The composition of aquarium water is often very different from one period to another or from one tank to another. It is subject to constant changes based on where it comes from (different water pipes, whether the water is “hard” or not, any blending or additions of water made with or without reverse-osmosis water, etc.), the amount of fish and plants in the tank, and the care and regular maintenance that have or have not been provided.
It is therefore important to check and monitor some key data on the water throughout the life of the tank, in order to correct any unfavourable changes in the chemical balance of the environment. Simple colorimetric tests and practices can be used to monitor the most important chemical parameters: pH, general hardness (GH) and carbonate hardness (KH)
pH
The pH or potential hydrogen (concentration of hydronium ions H3O+) is used to measure the acidity or alkalinity of water. In a test, neutral water is at pH 7, on a scale of 0 to 14.
For the majority of freshwater aquatic species (fish, plants and micro-organisms) the optimal pH value for the tank is between 6.8 and 7.5 pH units.
However certain species or situations might call for other ranges: Discus prefer the rather acidic pH 6.2; pH 8.1, somewhat basic, is better for some African Cichlidae; a pH of 8.2 to 8.5 is quite basic, but ideal for a seawater aquarium.
Plan on regular tests, at least every 2 months. The pH test is a very simple, comparative colorimetric test, very easy to do yourself.
GH (or TH)
A test of the hardness of the water, the GH test (used in the UK, USA, Germany and elsewhere) and the TH (used in France), is a measure of the concentration of mineral salts dissolved in the water [calcium (Ca2+) and magnesium (MG2+) cations]. Water can be “soft” (low mineralization) or “hard” (high mineralization). The GH value influences the metabolism of all animals and plants (the principle of osmoregulation).
The GH test measures the general water hardness: 1 drop of reagent before colour change is equal to 1 degree GH.
Note: the majority of tests on the market are in degrees GH; some countries use somewhat different measurements (eg, France uses “TH” degrees). To convert the GH degrees to TH degrees (reference to a French bibliography), multiply the GH result by 1.8 (example: 10° GH = 18° TH).
The GH measurement concerns only freshwater, since saltwater is supersaturated in a variety of inorganic salts.
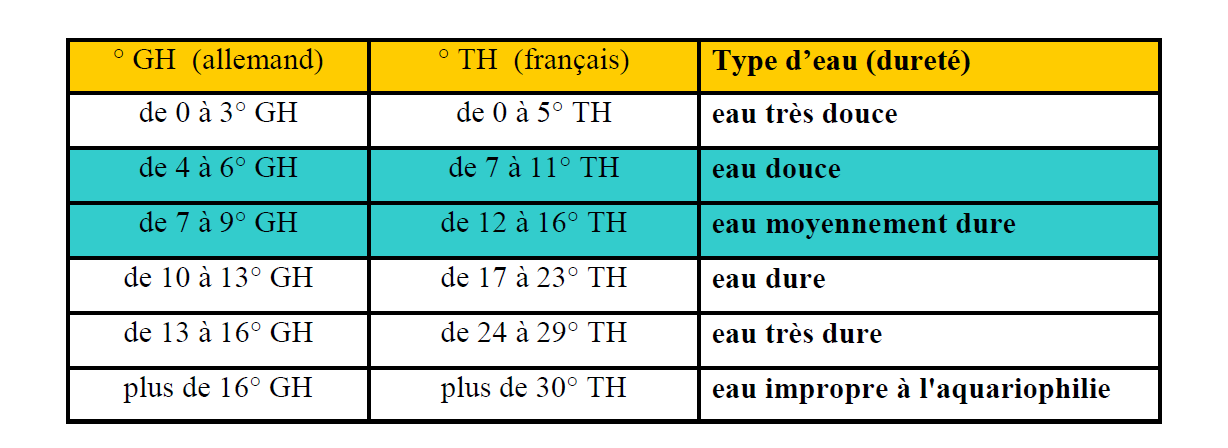
Water classification in relation to their hardness GH and TH
What should be done if the GH value is too high?
In an aquarium, the value should be within a range of 4 to 9 GH.
- If the test result is higher, eg, 14° GH, it will be necessary to replace 50% of the aquarium water with 50% demineralized or reverse osmosis water. The result will approach 7° (equivalent to moderately hard water).
- If the test result is less than 4° (very soft water), it will be necessary to add a small amount of minerals with a product such as GH+ (marketed to specialists and designed to remineralize R-O water so as to be suitable for aquarium use).
KH (or TAC)
Carbonate hardness or temporary water hardness.
KH is a very important factor in the aquarium. The carbonate hardness is the measure of the concentration of carbonate and bicarbonate anions; these salts act as buffer substances and are essential to the balance and stability of aquarium water; they help counteract sudden changes in pH.
If you need to convert GH degrees to TAC degrees (the French unit of measure – refer to a French bibliography), multiply the KH result by 1.8 (example: 10° KH = 18° TAC).
What should be done if the KH value is too high?
In an aquarium, to ensure a stable pH, the KH value should be within a range of 4 to 9° KH..
- If the test result is higher, say, 12° KH, it will be necessary to replace 50% of the aquarium water with 50% demineralized or reverse osmosis water. The result should then approach 6° KH.
- If the test result is less than 4° KH (very soft water), it will be necessary to add a small amount of minerals with a product such as KH+ (marketed to specialists and designed to remineralize R-O water so as to be suitable for aquarium use).
Iron Testing (Micronutrients for Plants)
Iron is a vital element in the physiology of fish but it is also essential for proper plant development.
Ferrous iron or ferric iron? In its “ferric” form, iron oxide (a rusty nail) is not very soluble in water and at this stage it is not available to plants. To be absorbed beneficially by plants and become a fertilizeing compound, ferric iron (Fe3+) must be associated with a chelating element (EDTA for example) to become “ferrous” (Fe2+), an ionic form that can be fully assimilated.
Ferrous iron, then, available as trace elements, is essential for the proper development and growth of plants.
Plan to test the iron content in the aquarium regularly and add a few drops of ferric fertilizer weekly to maintain a constant value between 0.05 and 0.10 mg/L.






